HOW TO MAKE A COIN BATTERY
Supplies you will need
6-8 Pennies (or more)
6-8 Nickels (or more)
Paper Towels or Coffee Filters
1/4 c. White Vinegar
1 Tbsp. Salt
Multimeter (voltage testing meter)
Small LED Pin Light
This coin battery will actually produce a voltage similar to a small battery! This coin battery makes about 1 volt of power.
Supplies you will need
6-8 Pennies
6-8 Nickels
Paper Towels
1/4 c. White Vinegar
1 Tbsp. Salt
Multimeter (voltage testing meter) and Small LED Pin

-mix together the salt in the vinegar until the salt is dissolved (in a small bowl).
Cut the paper towel into small squares. You will want them smaller than the coins so they are not overlapping.

Dip the squares into the vinegar and salt mixture and layer the coins in a pattern: penny, paper, nickel.
Once you have a large stack, you can test it out with your multimeter. You will want to turn it to a low voltage setting and test it out. To light up an LED light, it needs to reach about a 2 V reading on the multimeter.
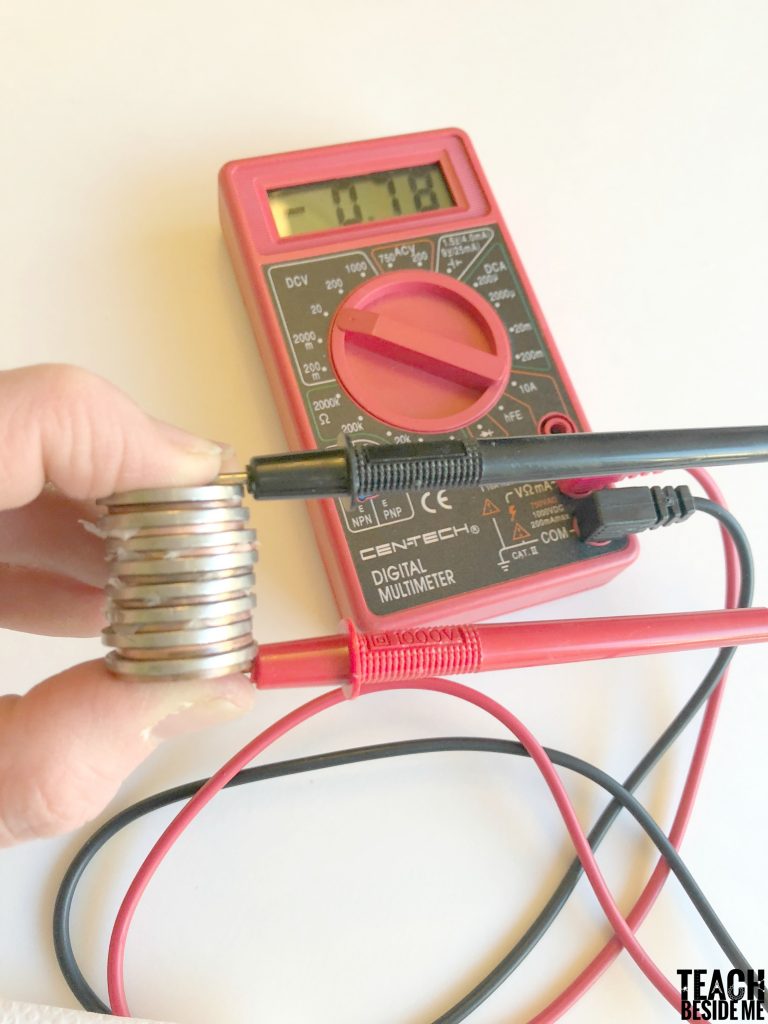
HOW DOES THE COIN BATTERY WORK?
-Nickels are made of zinc and pennies are made of copper. They are both conductors of electricity.
-When two different metals are connected by an electrolyte (which is the vinegar and salt solution), a chemical reaction occurs at the surface of the metals.
– These metals are the electrodes. When these electrodes are connected by a wire, they create an electrical current.
Source: teachbesideme.com
![]()
